OMEGA-3 AND QUALITY: how can you be sure of selecting a premium plant-based DHA?
Purification and preservation of strains
Isolating strains
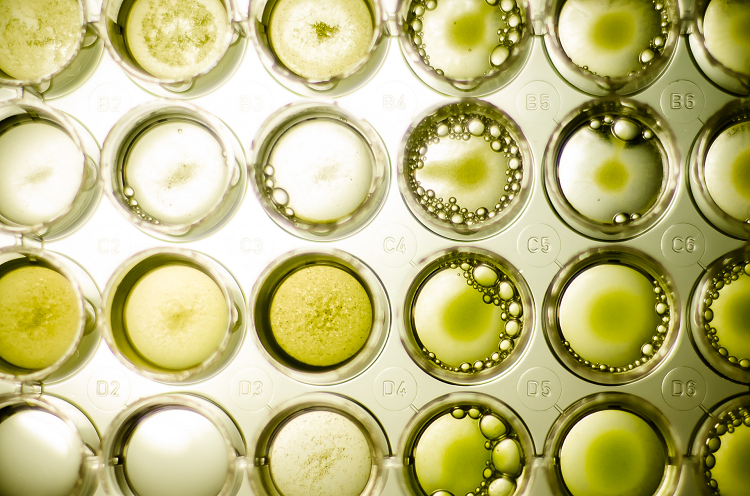
Environmental samples can contain a wide variety of microorganisms and the challenge is to isolate and purify the best. After incubation at temperatures similar to those found in the harvesting sites, several different types of colonies were seen to be growing. Some were potentially the Thraustochytriaceae that we wanted, but others were other types of microorganisms such as fungi or bacteria.
While Thraustochytriaceae grow quickly and this helps to isolate them, some of the contaminants in the samples can also grow quickly and this makes the task of target strain isolation and axenization[2] a complex one. The isolation stage essentially involves several subculturing techniques that allow us to separate the strains we want from those we don’t and can go as far as separating individual cells under the microscope.
Several successive subcultures[1] had to be prepared under a stereoscopic microscope, using such micromanipulation techniques and others to isolate and purify the strains we were interested in until we were sure that the strains were the only microorganisms growing. These techniques take days or even weeks before we obtained contaminant-free cultures, termed axenic. Since then the strains have been maintained using the best sterile technique to ensure that they remain pure and uncontaminated by other microorganisms.
Genetic identification
Inspection under a microscope examing the life cycle of the strain and genetic analysis subsequently confirmed the identity of each isolate[2].
In order to confirm the precise identity of the axenized strains, gene sequencing of several key reference genes was carried out. These key genes are found in almost all cells and vary just enough between species (not too much, not too little) that they can be used to generate family trees of organsims. Through comparison with the same gene sequences from other organisms contained in databases such as the NCBI (National Center for Biotechnology Information), we were able to precisely identify the genus and species of each isolate among the microalgae species collected with a number of good candidates in the Schizochytrium genus that we had targetted.
Storage in the Fermentalg Culture Collection
The axenized strains were delivered to the team responsible for the Fermentalg Culture Collection. Their aim is to preserve new strains freshly extracted from their natural medium to avoid the random changes in growth or composition that can sometimes be seen if strains start to adapt to the laboratory environment, which can be significantly different from nature. Each strain is allocated a unique number and kept by successive subculturing on a specified solid growth medium chosen to minimize the chance of change.
Cryopreservation storage (at -150°C) is a way of freezing the metabolism completely and thus preventing any changes in the strain since this only occurs as the cells divide and grow. It also has the advantage of reducing the need for subculturing and the risk of recontamination. Storage via cryopreservation is not as simple as just placing the cells in the freezer, and a freezing protocol has to be designed specifically for each strain using a programmable freezer that controls the rate at which the temperature drops. A good protocol ensures that the strains are viable after thawing and behave exactly as they did before freezing.
- Fermentalg has been engaging with stakeholders in the health market to offer them a wide portfolio of biosourced ingredients.
- Beyond its biotechnology mission, Fermentalg guarantees the preservation of existing natural resources by securing our living heritage, with a view to ensuring biodiversity for future generations.
Screening, characterization and development of strains
At this stage, Fermentalg had a number of candidates for DHA production, but the growth and biosynthesic capabilities were unknown. The next step was therefore to test different growth environments in order to identify the optimum growth conditions for DHA production.
- The trophic characteristics[3] were first studied in Erlenmeyer flasks exposed to light or kept in darkness, looking at how different organic carbon sources, nitrogen sources and other macro and micro elements were used and affected the production of DHA.
- Speed of growth was also measured and provided an initial comparison point between each strain.
- Biochemical analysis of the biomass composition was key to understanding the different strains of Schizochytrium sp. not just the lipids and fatty acids, but other classes of molecules such as pigments, polysaccharides and proteins.
- The industrial potential of each strain could then be assessed based the data relating to growth, DHA content and lipid accumulation, and how this would impact the production process and eventual product quality.
Our understanding of the basic biology of these types of organism has allowed us to encourage them to developed in ways that increase their suitability for industrialization. We have fine-tuned the conditions to naturally select strains that have faster growth and better omega-3 production proprieties than the initial strains did, in particular strains that produce higher proportions of DHA in their oils.
- Thanks to years of experience in microalgae characterization, Fermentalg has been able to identify a unique source of omega-3, the Schizochytrium sp. species used in the production of DHA ORIGINS®
- The exceptional natural yield of this microalga along with its nutritional content make it a genuine plant-based, ethical alternative to fish oils for laboratories.
[1] Microbiological subculturing means taking some microbes from a growth medium and transplanting them into a fresh growth medium
[2] Colony of microorganisms isolated from other organisms that may be present in the growth medium
[3] The nutrition and development conditions of organisms